COMPARING ADULT HUMAN PRIMARY CARDIOMYOCYTES TO HUMAN IPSC-DERIVED CARDIOMYOCYTES
Pre-clinical drug development continues to pose significant challenges for the pharmaceutical industry. The preclinical assessment of acceptable risk/benefit ratio, and the consequent selection of drug candidates worthy of further clinical studies, remains a major challenge.
Historical data shows that less than 15% of all clinical candidates receive regulatory approval.(1) Part of the challenge lies in the underlying differences between the species used as models in preclinical studies and humans. As a result, 75% of drug candidates terminated due to safety or efficacy issues were not accurately predicted by animal models.(2)
AnaBios’ advanced human tissue-based methods are enabling a modernized approach that integrates animal models with human ex vivo studies. This provides a very powerful strategy that combines the benefit of in vivo measurements (in animals) with the ability to generate human data (ex vivo) to overcome the challenges of cross-species translation.
PRIMARY HUMAN CARDIOMYOCYTES: BETTER TRANSLATION
In the late 1990’s, novel preclinical models based on human induced pluripotent stem cells (iPSC’s) were introduced. However, after more than two decades of research, human iPSC-derived models have not delivered on the early hopes and promises. Generally, these cell models lack key phenotypic features of adult human cells and express an immature phenotype.
In the context of studies aimed at establishing the cardiac safety of new drugs, iPSC-derived cardiomyocytes lack key features of human adult cardiomyocytes. (3,4) The adult cardiac muscle is formed by a syncytium of rod-shaped cardiomyocytes with highly organized, aligned sarcomeres. In contrast, stem cell-derived cardiomyocytes have a disorganized sarcomere structure which resembles the one observed in fetal and neonatal cardiomyocytes.
In the human heart, cardiomyocytes are paced in response to stimulations initiated by the sinoatrial node and do not beat spontaneously in the absence of external pacing. Consistent with this observation, isolated human cardiomyocytes only contract in response to external electrical stimulation.(5)
In contrast, human iPSC-derived cardiomyocytes beat spontaneously similarly as isolated neonatal ventricular cardiomyocytes. This discrepancy highlights key differences in the intracellular calcium dynamics between the two cell types which result in important differences in pharmacological responses.
In addition, both the absolute–as well as the relative level of expression of critical ion channels–differs between adult human primary cardiomyocytes and human iPSC-derived cardiomyocytes, providing additional opportunities for divergent pharmacology between the two cell types.
Table 1 lists the important differences between stem cell-derived cardiomyocytes and adult human primary cardiomyocytes, including cell maturity, structure and ion channel expression.
Table 1
PARAMETER | HUMAN STEM CELL-DERIVED CARDIOMYOCYTES | ADULT HUMAN PRIMARY CARDIOMYOCYTES |
|---|---|---|
Cell population phenotype | Mixed/Unreliable | Pure/Reliable |
Cell maturity | Immature | Adult |
Cell shape morphology | Irregular | Rod-shaped |
Sarcomeric structure | Disorganized | Organized |
T-tubule structure | Deficient | Abundant |
Ryanodine receptor distribution | Heterogenous | Homogenous |
Contraction force | Reduced | Significant |
Contraction | Spontaneous | Electrical pacing |
Ion channel expression | Under- or over-expressed | Physiological |
Ca2+ handling | Functional yet immature | Functional fully mature |
Force-frequency relationship | Negative | Positive |
Post-rest potentiation | Absent or weak | Present |
EXCITATION-CONTRACTION COUPLING
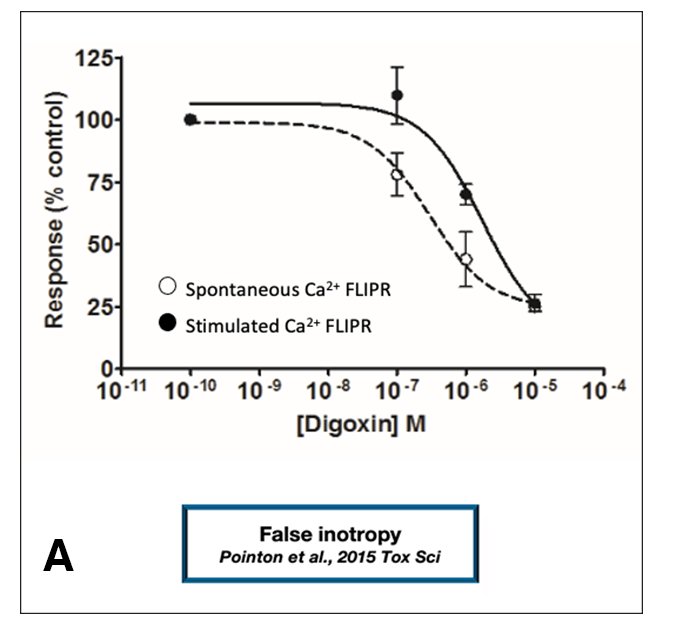
In addition to structural and electrophysiological differences, excitation-contraction (EC) coupling in adult human cardiomyocytes differs from that of human iPSC-derived cardiomyocytes. These EC coupling differences lead to differences in inotropic responses. A closer examination of the contractile properties of stem cell-derived cardiomyocytes reveals that these cells lack mature inotropic mechanisms (Figure 1). Using an intracellular calcium transients assay, Pointon et al. demonstrated that human iPSC-derived cardiomyocytes can exhibit false inotropy (Figure 1A).(6)
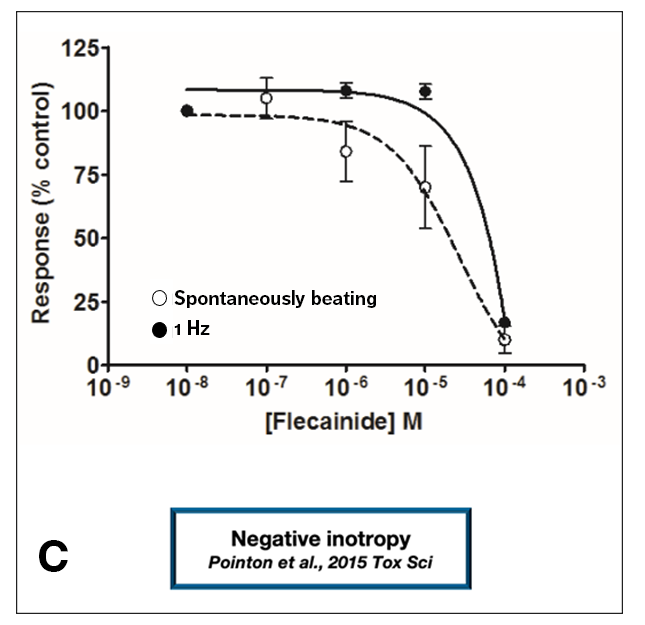
This study also provided an example of “false margin”; Figure 1C shows an example of negative inotropy in human iPSC-derived cardiomyocytes. The IC50 value for the observed inotropy effect is right-shifted when compared with the IC50 for negative inotropy measured by AnaBios in experiments using human primary cardiomyocytes.
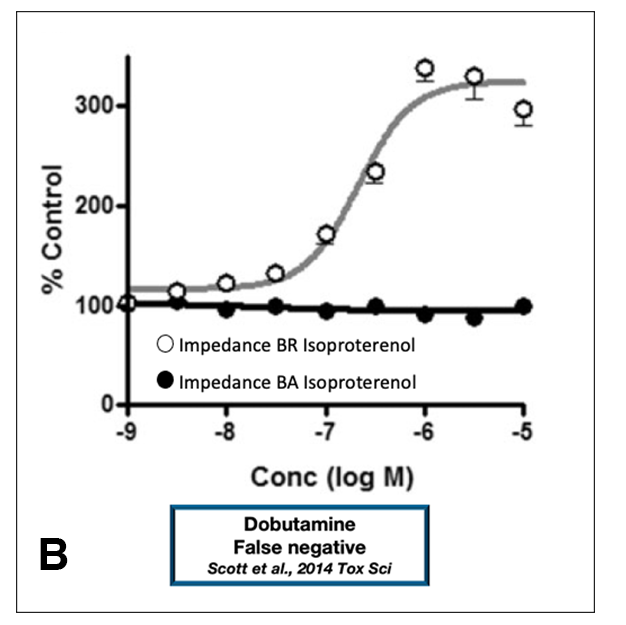
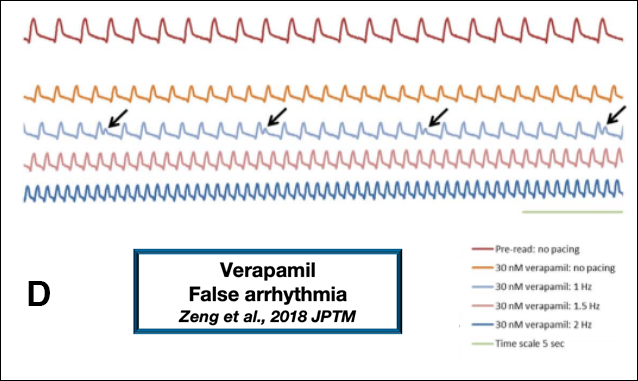
A study of risk assessment focused only on human iPSC-derived cardiomyocytes may inaccurately conclude that an adequate safety margin exists, hence the term “false margin.” Other studies have shown that false negatives (8) and false prediction of arrhythmia (9) can be generated when relying on these models (Figure 1B and 1D, respectively). Therefore, caution should be exercised when considering the use of human iPSC-derived cardiomyocytes for the study of drug-induced pro-arrhythmia and changes in contractility. (11)
Figure 1
DOWNLOAD CARDIOPRIME BROCHURE
To learn more about CardioPRIME, our unique human primary cardiomyocyte-based assay designed to simultaneously assess pro-arrhythmia and inotropic risk, please complete the form below and click the “Submit” button. You will be re-directed to our cardiac research library.
*By Clicking On The “Submit” Button Above, You Are Agreeing To AnaBios’ Privacy Policy.
ADULT HUMAN PRIMARY CARDIOMYOCYTES
RELIABILITY OF ADULT HUMAN CARDIOMYOCYTE DATA
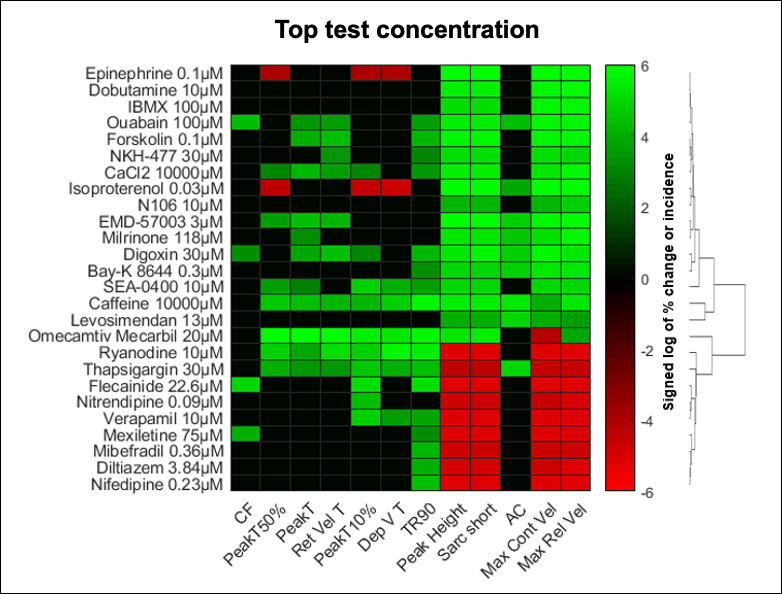
Adult human primary cardiomyocytes provide a reliable and predictive system for the study of drug-related pro-arrhythmia and contractility risks. AnaBios scientists used multiparametric mechanistic profiling of 17 positive inotropic drugs and nine negative inotropic drugs to show that these drugs caused concentration-dependent increases and decreases in sarcomere shortening, respectively.(9) This set of reference compounds covered 14 different mechanisms of action.
When testing drug candidates for pharmaceutical partners, AnaBios uses a heat map like the one in Figure 2 to profile the mechanism of action of a drug in adult human primary cardiomyocytes.
FIGURE 2
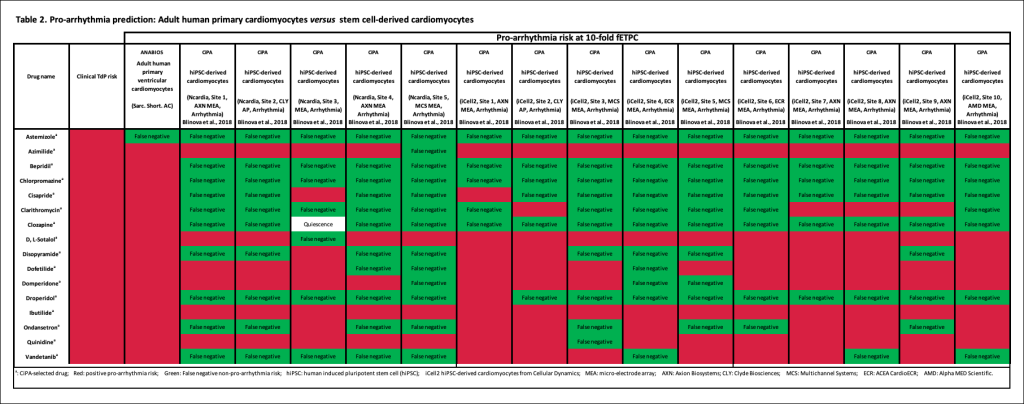
In a previous study,(5) AnaBios also showed that adult human primary cardiomyocytes predict the pro-arrhythmic risk of known clinical reference drugs with high sensitivity (96%). On the contrary, published results from various sources using stem cell derived cardiomyocytes indicate that this artificial model of human myocytes lacks phenotypic stability and is not a reliable predictor of pro-arrhythmia risk (Table 2, click image for larger version).
Moreover, these studies demonstrated 100% specificity with adult human cardiomyocytes when testing non-pro-arrhythmic drugs while results from iPSC-derived cardiomyocytes exhibited limited specificity (Table 2, click image for larger version). Together, these results demonstrate the superiority of AnaBios’ human adult primary cardiomyocyte platform and the measurement of parameters that are highly predictive of clinical outcomes. Click here to view our ethics statement.
TABLE 2
REFERENCES
2. Arrowsmith, J. & Miller, P. “Phase II and Phase III attrition rates 2011–2012”, Nature Reviews Drug Discovery, 12, 569, 2013
3. Parikh S. S., Blackwell D. J., Gomez-Hurtado N., Frisk M., Wang L., Kim K., Dahl C. P., Fiane A., Tønnessen T., Kryshtal D.O., Louch, W. E. & Knollman, B.C. “Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes”, Circ Res., Dec 8;121(12):1323-1330, 2017
4. Trieschmann et al., “Stem Cells Int Different Responses to Drug Safety Screening Targets between Human Neonatal and Infantile Heart Tissue and Cardiac Bodies Derived from Human-Induced Pluripotent Stem Cells,” 2019
5. Nguyen, N. al., “Adult Human Primary Cardiomyocyte-Based Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-arrhythmia Risk”, Front. Physiol. 8:1073. (doi: 10.3389/fphys.2017.01073)
6. Pointon, A. et al. Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes. Sci.144(2), 227–237 (2015).
7. Scott, C.W. al., “An Impedance-Based Cellular Assay Using Human iPSC-Derived Cardiomyocytes to Quantify Modulators of Cardiac Contractility”, Tox. Sci., Volume 142, Issue 2, December 2014, Pages 331–338 (https://doi.org/10.1093/toxsci/kfu186)
8. Zeng, H., Balasubramanian, B., Lagrutta, A. & Sannajust, F. Response of human induced pluripotent stem cell-derived cardiomyocytes to several pharmacological agents when intrinsic syncytial pacing is overcome by acute external stimulation. Pharmacol. Toxicol. Methods91, 18–26 (2018).
9. Abi-Gerges, N. al., “Multiparametric Mechanistic Profiling of Inotropic Drugs in Adult Human Primary Cardiomyocytes”, Scientific Reports, 10, 7692 (2020). https://doi.org/10.1038/s41598-020-64657-2.
10. Blinova, K. et al. Comprehensive translational assessment of human induced pluripotent stem cell derived cardiomyocytes for evaluating drug-induced arrhythmias. Tox Sci.155(1), 234–247 (2017).
