About Us
It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout.

It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout.

Clinically relevant cardiac physiology
Direct measurement of cardiac function
Inotropy, electrophysiology and calcium signaling
Identify liabilities sooner
CardioPRIME is the AnaBios approach for measuring human cardiomyocyte functionality and sets the industry standard for cardiac-based biomedical research. By leveraging the robust excitation-contraction coupling of mature primary human cardiomyocytes and direct measurements of sarcomeric length, CardioPRIME offers unprecedented insight into cardiomyocyte functionality and provides clinically translatable measurements and biomarkers for proarrhythmia, inotropy, Ca2+ signaling, electrophysiology and cell health.
As shown in the video below, adult primary cardiomyocytes dissociated from healthy human hearts are placed into optical recording chambers and stimulated at predefined frequencies. Sarcomeric shortening is measured and shown as deflections from baseline.
CardioPRIME harnesses fully functional human cardiomyocytes. The video above illustrates the CardioPRIME platform. Dissociated adult human primary cardiomyocytes are paced via extracellular field stimulation and the resulting sarcomeric shortening is measured. In this example, the cardiomyocyte is paced at 0.5, 1 and 2Hz and the resulting positive frequency-contraction relationship demonstrates the fully functional excitation-contraction process with physiological calcium handling and electromechanical coupling; processes that are often absent or limited in other cardiac models.
Proarrhythmia detection with CardioPRIME leverages the tight coupling between electrical events at the membrane and contraction and is illustrated in the figure below. Agents that prolong the action potential and/or cause proarrhythmogenic events such as early afterdepolarizations (EADs) are detected as prolongations and after contractions (AC) in the contractility waveform, respectively. Using these and other waveform-based biomarkers, Nguyen and colleagues demonstrated an industry-leading predictivity rate of 96% for detecting proarrhythmia (Nguyen et al., 2017).
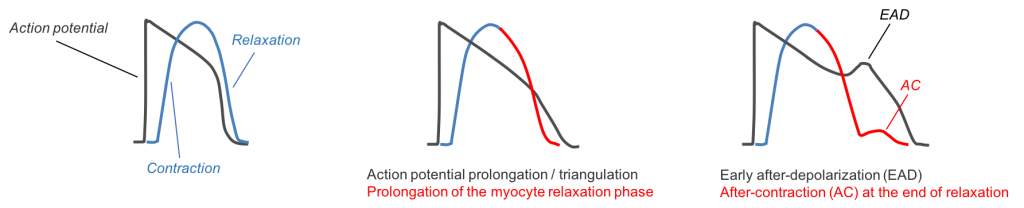
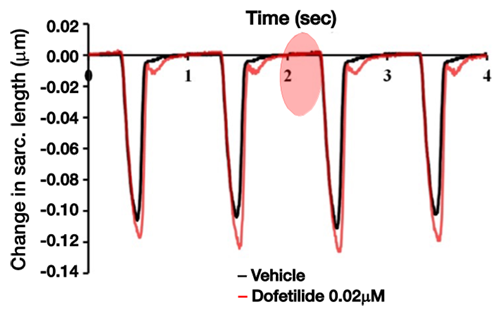
Figure 1: Top image, left panel: The action potential drives physical contraction and relaxation. Top panel, middle: Changes in the shape of the action potential, such as drug-induced prolongation, causes subsequent changes in the contractility waveform. Top panel right: Sentinel proarrhythmic triggers such as early-afterdepolarizations (EAD) are reflected in the contractility transient as after-contractions (AC).
Figure 1: Lower image: Real-world example of detecting proarrhythmia as demonstrated by the application of dof. (0.02µM) and AC generation (adapted from Nguyen et al., 2017).
Modulating cardiac inotropy is a highly sought-after therapeutic endpoint for heart failure treatment while the appearance of unanticipated inotropic effects in other discovery efforts can halt clinical development.
As shown in Figure 2 below, CardioPRIME and primary human cardiomyocytes can be used to detect both positive and negative inotropy across multiple mechanisms for both therapeutic drug discovery and safety testing. Vice President of Research & Development Dr. Najah Abi-Gerges and his colleagues recently write a publication for more complete analysis of detecting positive and negative inotropy (Abi-Gerges et al., 2020).
The ability of CardioPRIME to reliably detect both positive and negative inotropic changes in primary human cardiomyocytes provides early insight into both efficacy and potential toxicities and offers clear translational value that overcomes limitations of stem cell models and avoids costly errors associated with cross-species translation.
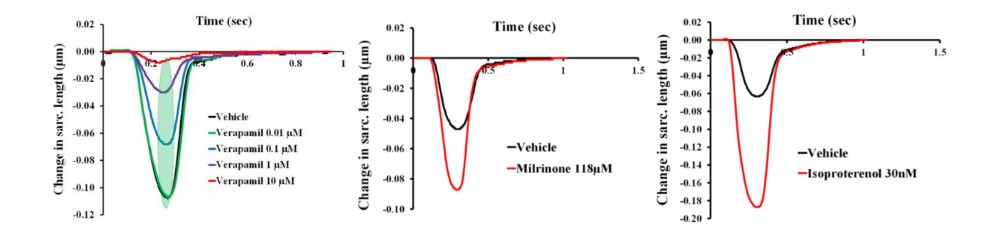
The calcium channel blocker ver. (left), which is known to reduce cardiac contractility through Ca2+ channel block, elicits negative inotropy while milr. (middle) and isop. (right) elicit positive inotropic effects through the phosphodiesterase and b-adrenergic systems, respectively.
MYOBLAZER is the proprietary higher throughput platform for CardioPRIME measurements across multiple cells at the same time. As illustrated in Figure 3 and 4 below, MYOBLAZER simultaneously visualizes, stimulates and analyzes multiple cardiomyocytes at the same time.
Simultaneous compound application and data collection reduces experimental time, minimizes time-dependent variability, and increases data robustness which offers a clear advantage over true contractility platforms that rely on measurement of individual cells in a linear fashion
MyoBLAZER enables rapid data collection and assessment. Multiple cells are imaged at once in the video in Figure 3 above. In Figure 4 below, regions of interest are defined (left image) and data is simultaneously collected across all denoted cardiomyocytes (right image).
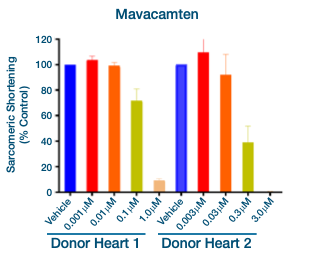
In Figure 5 above, cardiomyocytes from two different healthy donors demonstrate decreased sarcomeric shortening (negative inotropy) in response to application of the myosin inhibitor Mavacamten.
What Is The Turnaround Time For Drug Testing In CardioPRIME?
CardioPRIME is a cell-based assay currently run on the MYOBLAZER™. The moderate throughput enabled by this platform allows for a turnaround time of approximately 4-6 weeks (depending on the service queue).
Do You Test Drug Effects In Cardiomyocytes From Different Donors?
Studies conducted at AnaBios have demonstrated that donor-to-donor variability in drug effects is very low when cells from hearts not affected by cardiac disease are used. It is therefore sufficient to rely upon cardiomyocytes from a single donor; this allows for a rapid turnaround time. When the possibility of donor-to-donor variability is a concern, it is possible to perform studies involving the use of cardiomyocytes from multiple donors.
What Is The Sensitivity And Selectivity Of CardioPRIME?
We have extensively validated CardioPRIME utilizing a set of 38 molecules with known clinical outcomes, previously selected by the CiPA and JiCSA initiatives. Based on these studies, we have reported a sensitivity of 96% and a specificity of 100% (see Nguyen et al., 2017 ).
How Is The Cell-Pacing Frequency Controlled?
CardioPRIME utilizes primary adult human cardiomyocytes. These cells do not exhibit spontaneous contractions and require external stimulation. Cells are paced at 1Hz via field electrodes.
Are Controls And Washouts Included In The Assays?
Relevant positive and negative controls and a washout period can be included in the study design.
What Is The Assay Format?
Baselines for all cells are obtained prior to cumulative concentration testing. Drug effects are compared to their determined baseline.
What Is The Drug Exposure Time In CardioPRIME?
A standard assay is five minutes per concentration. The overall duration of the assay is about 20-30 minutes, including baseline control. The time can be allocated to the testing of multiple concentrations or extended testing of a single concentration.
Which formulation vehicles are compatible with CardioPRIME?
Drugs can be formulated in saline buffer or in buffer containing 0.1% to 0.3% DMSO.
Cardiomyocyte
Muscle cells that make up the heart and are responsible for contractions necessary for blood circulation.
Sarcomere
The basic functional structure of striated muscle cells. Sarcomeres are composed of long, fibrous actin and myosin filaments that slide past each other when a muscle contracts or relaxes.
Drug-Related Pro-Arrhythmia Risk
The risk of a drug to induce irregular contractions in the heart. Drug-induced arrhythmias can cause ineffective cardiac contractions and reduced cardiac output, which can lead to serious, and even fatal, adverse events.
Drug-related inotropic risk
The risk of a drug to induce changes in cardiomyocyte contractility. This can result in reduced contractility (negative inotropy) or enhanced contractility (positive inotropy). The former can lead to dangerously low cardiac output, while the latter can lead to cardiac overexertion and possibly heart failure.
Electromechanical coupling
The mechanistic coupling of electrical excitability and contractile activity in cardiomyocytes. Following an initial depolarization, once a sufficient number of plasma membrane voltage-gated sodium channels are activated, an action potential is initiated in a cardiomyocyte. The resulting depolarization activates the voltage-gated calcium channels. The influx of calcium ions further triggers calcium release from the sarcoplasmic reticulum. The increase in cytoplasmic calcium activates the contractile machinery, leading to sarcomere shortening and cardiomyocyte contraction.
Early afterdepolarization (EAD)
The premature and abortive initiation of a new action potential, often resulting from a sustained cardiomyocyte depolarization and reactivation of voltage-gated sodium and/or calcium channels. It can lead to desynchronization of cardiac action potentials and abnormal secondary contractions across different regions of the heart, potentially resulting in life-threatening arrhythmias.
Aftercontraction (AC)
An abnormal secondary contraction initiated by a cardiomyocyte in the absence of external stimulation. Aftercontractions can be the mechanical consequence of EAD and/or compromised intracellular calcium homeostasis, resulting from sustained and abnormal cytoplasmic calcium levels.

To inquire about products, services and pricing, please go to the ‘Contact Us’ page by clicking the button below.