PAIN RESEARCH WEBINARS
AnaBios offers a number of pain research webinars from top pharma and academic researchers across the globe. Click the blue button be
A Novel Approach to Utilizing AI & Biological Assays to Help Address the Opioid Crisis
Presented by Dr. Jeffrey Skolnick & Dr. Andre Ghetti
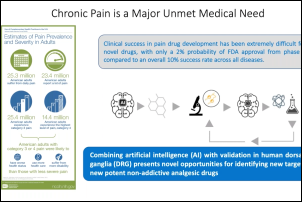
To help address the opioid crisis, the development of better small molecule treatments for pain with minimal addiction propensity or drug overdose is a pressing need. To accelerate the discovery of such ideal small molecules, AnaBios and Georgia Tech University have partnered to develop a novel strategy which comprises three components: a predictive machine learning algorithm, an electronic Synthetic Chemistry Portal infrastructure and a novel biological assay to validate their efficacy. The machine learning algorithm was trained to identify molecules with analgesic potential and furthermore, flag compounds with heightened risk for abuse/dependence side effects. The resulting diverse small molecule libraries with predicted low addiction potential were selected for testing of the biological effects in human sensory neurons in culture. The webinar presents data from a small proof of concept study which illustrates the potential of the novel approach.
High-Depth Single Soma RNA of Human DRG Neurons
Presented by Dr. Wenqin Luo
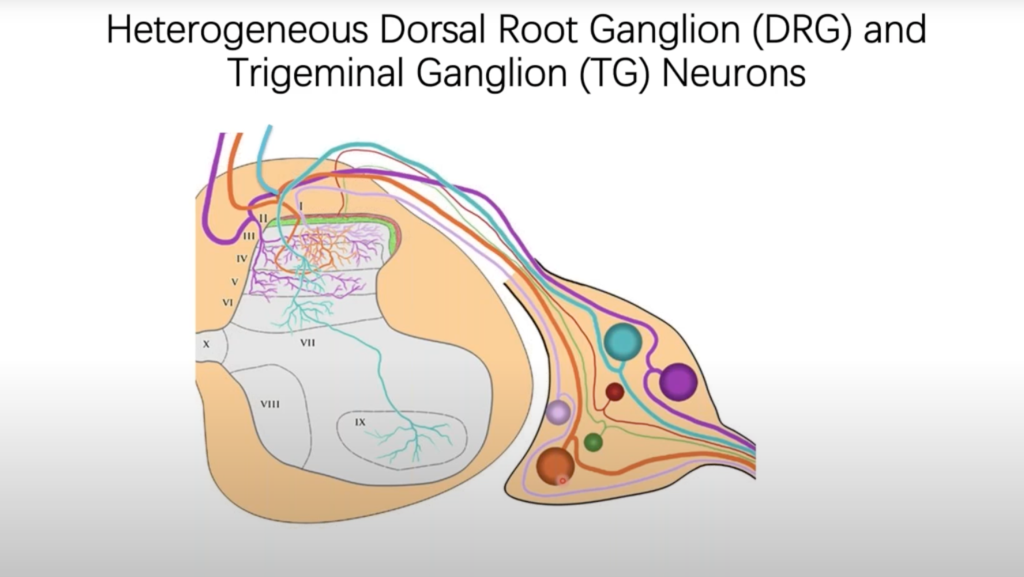
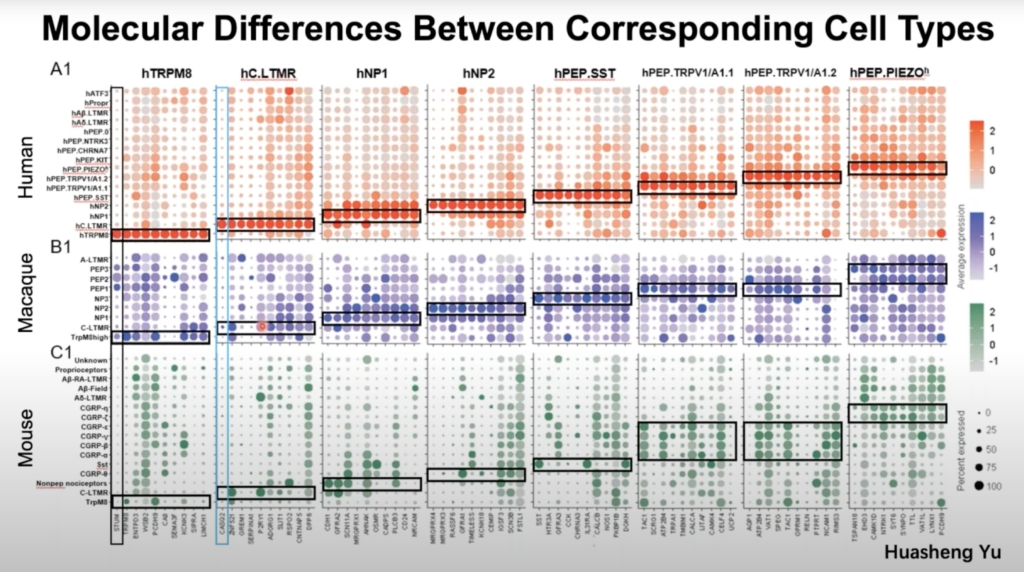
Most knowledge regarding dorsal root ganglia neurons comes from model organisms, which may have significant species differences from humans. Here, Dr. Wenqin Luo’s research team developed a new approach by isolating individual somas of human DRG neurons using laser capture microdissection and performing high-depth Smart-seq2 RNAseq. Their results not only provide novel molecular and cellular mechanisms for understanding human somatosensation but serve as an important reference when translating animal studies into human treatments. Click here to view the preprint of Dr. Luo’s research.
Strategies to Leverage Human Tissue for Pain & Analgesia Studies
Presented by Dr. Michael Gold
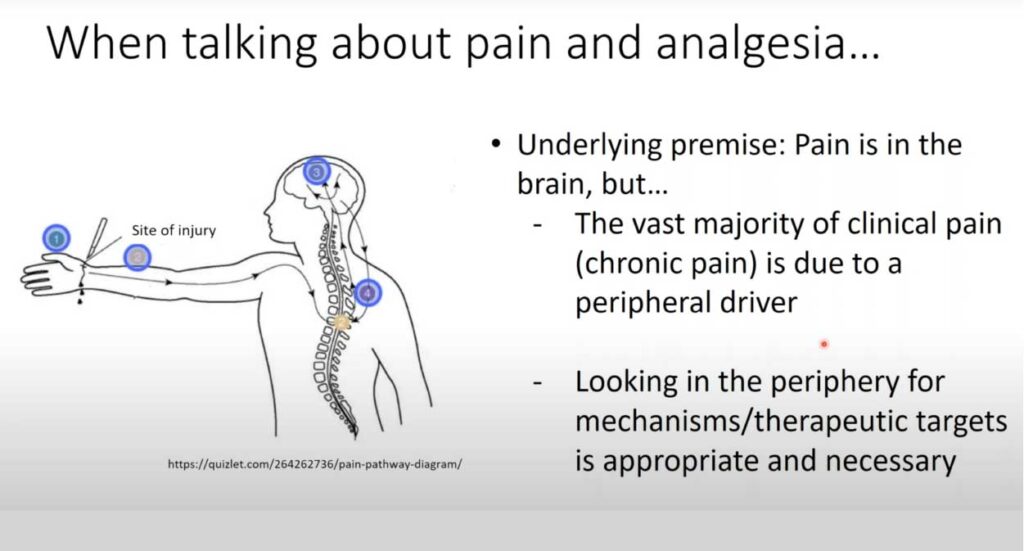
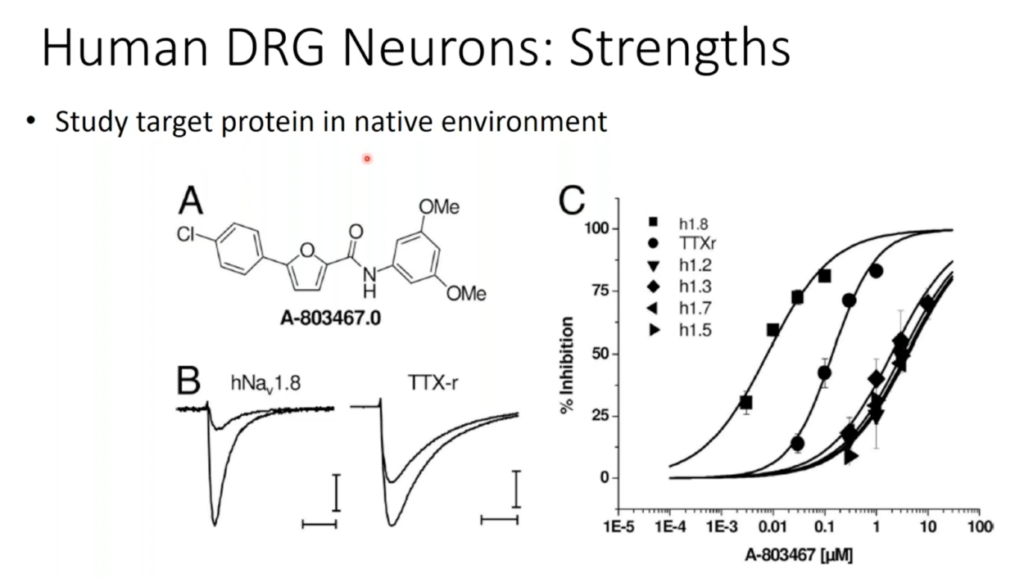
In this 60-minute video, Dr. Michael Gold, Professor of Neurobiology at the University of Pittsburgh, presents new strategies to leverage human tissue and cells for pain and analgesia research. The purpose of this presentation is to remind investigators that there are many sources of tissue that may be used in the study of mechanisms of pain and analgesia. The focus of Dr. Gold’s research is the neurobiology of pain. He has made important contributions to injury-induced plasticity in nociceptive afferents, and their contribution to the manifestation of persistent pain. Toward this end, he has employed an array of approaches ranging from the study of isolated cells to the development of novel behavioral assays with which to assess the presence of persistent hypersensitivity, and more recently the study of clinical populations suffering from persistent pain.
Painful Diabetic Neuropathy: Single-Cell RNA Sequencing of Peripheral Somatosensory Neurons
Presented by Dr. Daniela Menichella
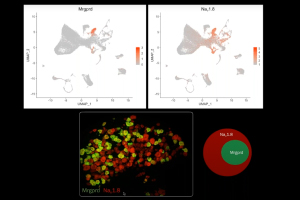
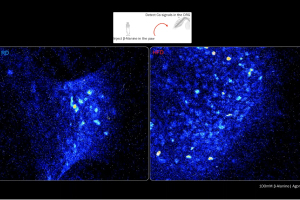
Dr. Daniela Menichella, Associate Professor of Neurology and Pharmacology at Northwestern University, discussed how an unbiased single-cell transcriptional profiling approach revealed the complete gene expression profile of molecularly distinct dorsal root ganglia (DRG) neuronal subtypes, along with the specific genes differentially expressed in individual neuronal subtypes under diabetic and non-diabetic conditions in the High-Fat-Diet mouse model of painful diabetic neuropathy (PDN).
Excitatory NMDA Receptors in Rodent & Human Spinal Pain Processing
Presented by Dr. Mike Hildebrand

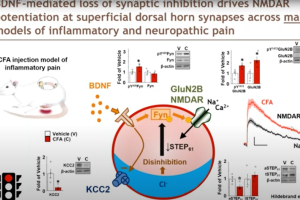
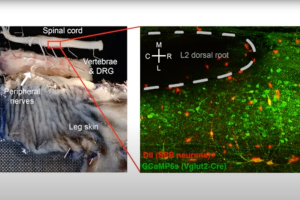
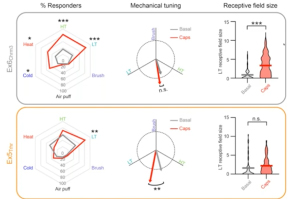
In this 60-minute video, Dr. Mike Hildebrand, Associate Professor of Neuroscience at Carleton University, highlights his team’s recent work developing new human spinal cord tissue models of pain processing that parallel rodent in vivo and ex vivo pain models. Using these complementary approaches, they are investigating molecular mechanisms of spinal pain processing across development, sex and species, including the function and regulation of excitatory glutamate receptors at dorsal horn synapses.
Dr. Sarah Ross, Associate Professor of Neurobiology at the University of Pittsburgh Medical School, describes her team’s approach using 2P Ca2+ imaging in the dorsal horn to investigate the neural circuits that integrate pain and itch. Viewers can expect to gain a clearer understanding of how nociception is integrated in the dorsal horn, insight into the cell types that convey this information from the spinal cord to the brain and how information processing changes in the context of injury.
Development of Mouse Pain Scales to Unlock Sensory & Motivational Pain
Presented by Dr. Ishmail Abdus-Saboor
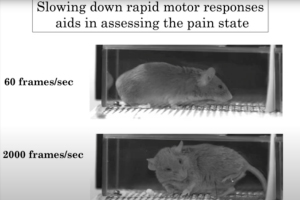
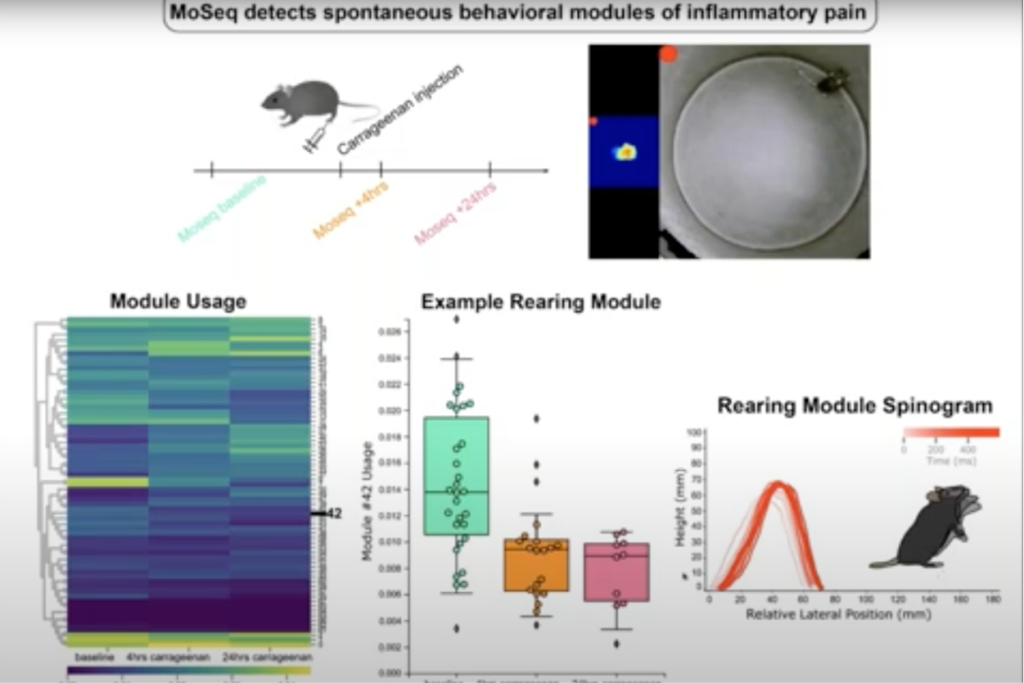
Ishmail Abdus-Saboor, PhD, Assistant Professor of Biological Sciences at the Zuckerman Brain Behavior Institute at Columbia University, demonstrated how he and his team uncovered a behavioral map of the sensory-versus-motivational components of pain in mice across timescales and dimensions using a combination of supervised and unsupervised approaches. Their findings reveal that sensation versus motivational drive can be uncoupled and objectively quantified, even in animals that cannot articulate these often hidden and personal dimensions of the painful experience.
Novel Human Spinal Cord Platform for Preclinical Drug Discovery
Presented by Dr. Michael Iadarola & Dr. Andre Ghetti
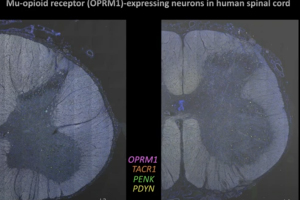
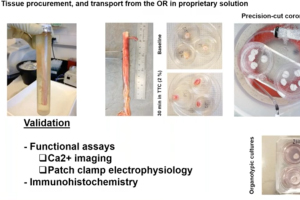
Dr. Andre Ghetti and Dr. Vivekanand Jeevakumar from AnaBios are joined by Dr. Michael Iadarola, Senior Researcher at the National Institute of Health (NIH), to present a new human spinal cord platform for preclinical drug discovery in this 60-minute webinar.
Translational Pain Research: Targeting Sensitization
Presented by Dr. Rob Gereau
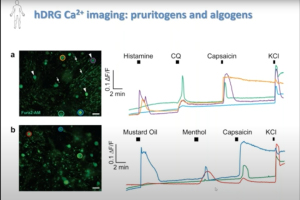
In this 60-minute video, Dr. Rob Gereau, Vice-Chair for Research at the Washington University Pain Center, describes preclinical studies in rodents identifying key nodes of sensitization in pain pathways that his research team is targeting for the development of potential new analgesics. He also discusses the use of human sensory neurons as a critical translational research tool.
Flexible Identity of Peripheral Sensory Neurons After Axonal Injury
Presented by Dr. William Renthal
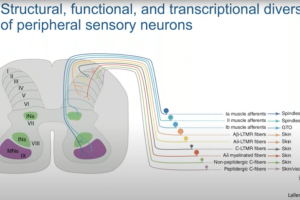
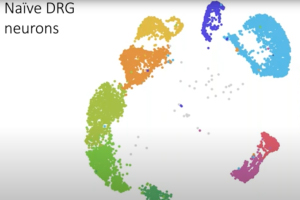
Dr. William Renthal, Director of Research at the John R. Graham Headache Center at Brigham and Women’s Hospital and Assistant Professor of Neurology at Harvard Medical School, presents our webinar, “Flexible Identity of Peripheral Sensory Neurons After Axonal Injury.” Primary somatosensory neurons are specialized to transmit specific types of sensory information. By profiling sensory ganglia at single-cell resolution, Dr. Renthal’s team discovered that all somatosensory neuronal subtypes undergo a transcriptional response to peripheral nerve injury that both promotes axonal regeneration and suppresses cell identity. This transcriptional reprogramming, which is not observed in non-neuronal cells, resolves over a similar time course as target reinnervation and is associated with the restoration of original cell identity.
Piezo2 & TRPV5 Channels: Study of the Endogenous & Exogenous Mechanisms
Presented by Dr. John Del Rosario
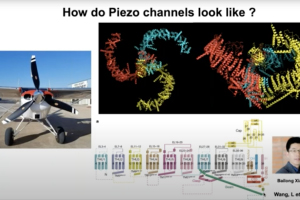
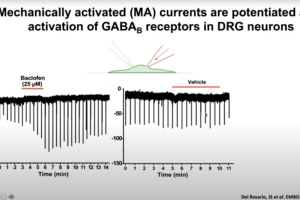
Dr. John Del Rosario, Postdoctoral Research Associate at Gereau Lab at the Washington University Pain Center, presents this webinar that examines the endogenous and exogenous mechanisms that modulate activity of Piezo2 and Ca2+- selective transient receptor potential vanilloid 5 (TRPV5) channels. Piezo2 channels have been identified as key players in mechanotransduction of light touch and proprioception in humans and mice. Dr. Del Rosario will explore the endogenous mechanisms that regulate the activity of Piezo2 after activation of Gi-protein coupled receptors. He will also discusses how exogenous modulators regulate TRPV5 channels, which are essential for calcium reabsorption and homeostasis in the kidney.
Bridging the Translational Divide:Rodent & Human Models of Pathological Spinal Pain Processing
Presented by Dr. Mike Hildebrand
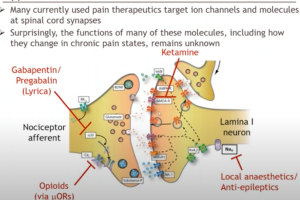
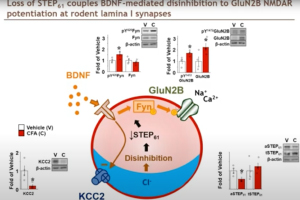
Dr. Michael Hildebrand, Associate Professor of Neuroscience at Carleton University, highlights his recent work aimed at bridging the translational divide between target identification in rodent models of chronic pain and direct clinical testing of candidate compounds in humans. His research team has developed a novel human spinal cord tissue model of pathological pain that parallels rodent in vivo and ex vivo pain models. Using these complementary approaches, Dr. Hildebrand and his team are identifying molecular determinants of spinal cord hyperexcitability that are conserved across species, and thus may inform the rational development of more effective therapeutics for chronic pain.
Chronic Orofacial Pain: From Where We Have Been to Where We Will Go
Presented by Dr. Carol Meloto

In this hour-long webinar, Dr. Carol Meloto, General Dentist and Assistant Dentistry Professor at McGill University, discussed how our understanding of the mechanisms underlying the risk of having and of developing temporomandibular disorders—the most frequent forms of chronic orofacial pain—have evolved in the past decade. She also discusses her current work focused on applying these recent advances to improve diagnosis and prognosis for chronic orofacial pain patients.
Chronic Low Back Pain & Epigenetics: Perspectives from Human & Animal Studies
Presented by Dr. Laura Stone
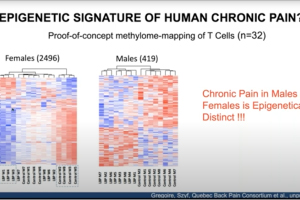
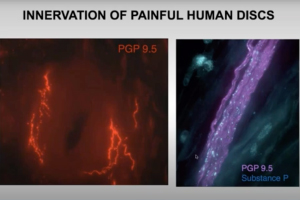
In this pain research webinar, Dr. Laura Stone, anesthesiology professor at the University of Minnesota, presents findings on low back pain that involve both human and animal models. She discusses dysregulation of nerve growth factor and immune mediators in human cerebrospinal fluid and intervertebral discs obtained from patients vs. controls. She also explores proof-of-concept studies targeting these mechanisms in a pre-clinical model of low back pain, and presents human and animal study data implicating epigenetic mechanisms in chronic pain.
Sickle Cell Disease Pain Mechanisms: From Mice to Humans
Presented by Dr. Cheryl Stucky
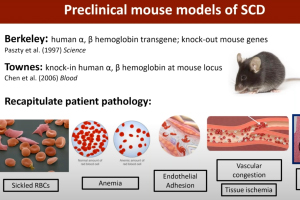
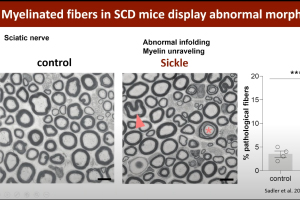
Pain is the chief morbidity that severely decreases the quality of life of patients who suffer from sickle cell disease (SCD). Many also suffer from chronic pain that may result from chronic inflammation and dysregulated neuronal firing and connectivity within the peripheral and central nervous system. In this webinar, Dr. Cheryl Stucky, Director of the Neuroscience Doctoral Program at the Medical College of Wisconsin, discusses her lab’s work interrogating peripheral mechanisms underlying sickle cell pain by using rodent models of SCD in combination with electrophysiological, cellular and behavioral assays. She also discusses her work with colleagues to investigate phenotypes and mechanisms of pain in patients with sickle cell disease.
Human Dorsal Root Ganglion Transcriptomics for Pain Mechanism Discovery
Presented by Dr. Ted Price
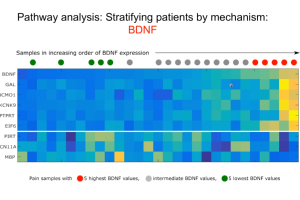
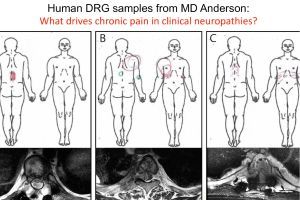
There is an urgent need for new therapeutics to address chronic pain, which affects approximately 20% of U.S. adults. This 60-minute webinar, presented by Dr. Ted Price, neuroscience professor at the University of Texas at Dallas, focused on utilizing human dorsal root ganglia (DRG) samples from patients and organ donors to gain new insight into the molecular mechanisms that may underlie chronic pain. Dr. Price also discusses integrating sensory neuronal transcriptomics with other datasets more widely available from patients to better understand how the nervous system interacts with diseased tissues to promote chronic pain. This unique approach offers opportunities to thoroughly vet pain targets without relying heavily on pre-clinical models.
Kappa Opioid Receptors in Chronic Pain & Associated Affective Disorders
Presented by Dr. Catherine Cahill
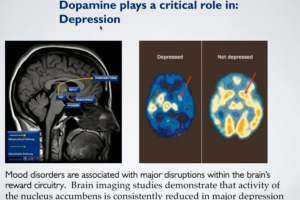
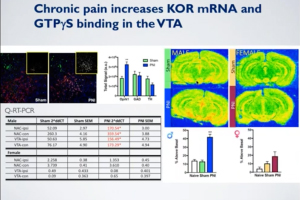
Stream “Kappa Opioid Receptors in Chronic Pain & Associated Affective Disorders,” presented by Dr. Catherine Cahill, Director of the Pain & Addiction Lab at the University of California, Los Angeles. Pain is composed of two essential processes: a sensory component that allows for discrimination of the intensity and location of a painful stimulus and an emotional component that underlies the affective, motivational, unpleasant and aversive response to a painful stimulus. Kappa opioid receptor (KOR) activation throughout the neuroaxis modulates both of these components of the pain experience. In this webinar, Dr. Cahill presented recent findings that KORs contribute to the emotional, aversive nature of chronic pain, including how expression in the limbic circuitry contributes to anhedonic states and components of opioid misuse disorder.
Nerve Function in Murine Models of Prediabetes
Presented by Dr. Amy Rumora
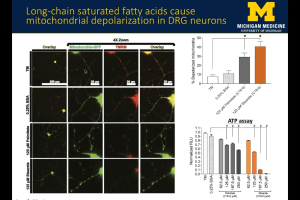
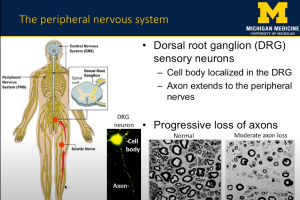
Peripheral neuropathy (PN) is the most common complication of type 2 diabetes (T2D) and prediabetes. Patients with peripheral neuropathy experience a length-dependent loss of sensation in their limbs due to peripheral nerve damage. The severity of PN in T2D and prediabetic patients correlates with dyslipidemia, indicating that dietary fatty acids contribute to PN pathogenesis. In this webinar, Dr. Amy Rumora, Postdoctoral Fellow at the University of Michigan School of Medicine, presents research that examines the impact of dietary saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) on nerve function in a murine model of prediabetes. The data suggests that SFA-induced mitochondrial dysfunction contributes to PN development, and that MUFAs restore nerve function and prevent mitochondrial dysfunction through lipid droplet formation.
Novel Tools for Translational Research: Ex Vivo Human Models for Advancing Drug Discovery
Presented by Dr. Andre Ghetti
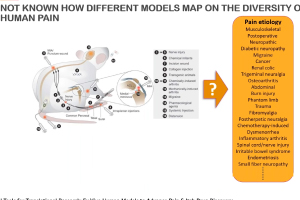
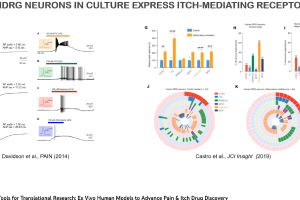
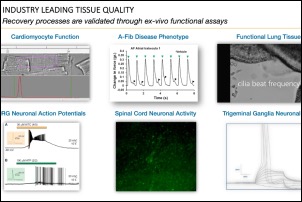
Dr. Andre Ghetti, CEO of AnaBios, presented “Novel Tools for Translational Research: Ex Vivo Human Models for Advancing Pain & Itch Drug Discovery,” as part of Neuroservices-Alliance’s ongoing webinar series. He highlighted AnaBios’ unique expertise in ex vivo studies of human pharmacolgy in normal and pathological states. He also discusses how this research can be used in the context of drug discovery targeting pain and itch. AnaBios provides ethically-sourced human tissue samples that have been processed utilizing proprietary methods to maximize the preservation of physiological function and help ensure success in experimentation involving functional end points or gene expression analysis, proteomics and metabolomics. Our human tissue quality control, based on measurements of physiological function, provides a unique guarantee of exceptional quality and provides the highest grade tissue for supporting scientific research and drug discovery.